In the modern healthcare ecosystem, clinical data management software has turned into a powerful tool for clinics and other healthcare organizations.
With growing regulatory expectations, decentralized trials, and the sheer volume of real-world data pouring in from multiple sources, clinical research teams are being pushed to a tipping point. And that’s precisely why Clinical Data Management Software (CDMS) has moved from being a “nice-to-have” to an “absolute must.”
But not all CDMS platforms are created equal. The question is not only about digitizing data anymore but also managing it with integrity, auditability, and real-time insights while reducing human error and operational drag.
This blog dives deep into the benefits, core processes, and cost of implementing a robust clinical data management system in 2025.
Whether you’re a biotech startup preparing for your first Phase I trial or a large pharmaceutical company optimizing your pipeline, understanding the full value and mechanics of a CDMS will help you move smarter, faster, and in compliance.
So let’s dive into
Table of Contents
What is Clinical Data Management Software?
The Clinical Data Management Software (CDMS) is the brain of modern clinical trials. It is a system managing or processing every piece of data collected during a clinical study so that it is accurate, validated, traceable, and regulatory-ready. Clinical trials nowadays have become sprawling ecosystems involving stakeholders—clinicians, CROs, data managers, statisticians, and regulators.
Without a tangible CDMS, trying to keep track of all the data streaming in from sites, wearables, labs, and remote patients would be akin to trying to build a skyscraper on quicksand.
With that said, a modern CDMS guarantees the confidence that clinical data has been curated, cleaned, queried and is ready for submission. More importantly, they diminish risks and minimize timelines, allowing stakeholders to make decisions in real time that could save years in drug development.
Key Features of Clinical Data Management Software:
- Electronic Data Capture (EDC)
- Data Validation & Query Management
- Audit Trails & User Access Logs
- Role-Based Access Control (RBAC)
- eCRF (Electronic Case Report Form) Designer
- Real-Time Data Monitoring & Reporting
- Multilingual & Multi-Site Support
- Data Export & Integration Tools
- Regulatory Compliance Management
- Mobile Accessibility
- Adverse Event (AE) & SAE Reporting
- Randomization & Trial Supply Management (RTSM)
Why is the Need for Clinical Data Management Software Increasing in Today’s Healthcare System?
In a precision-driven, data-obsessed world of healthcare developments, relevant observations are made on hard, high-quality evidence. As the volume, velocity, and variety of healthcare data explode, the clinical data management software became mission-critical.
According to Precedence Research, the global clinical data management system market size recorded $3.46 billion in 2025, which is now projected to attain $8.90 billion by 2034 at a CAGR of 11.09% between 2025-2034. In North America alone, the clinical data management system market size exceeded $1.43 billion in 2024.
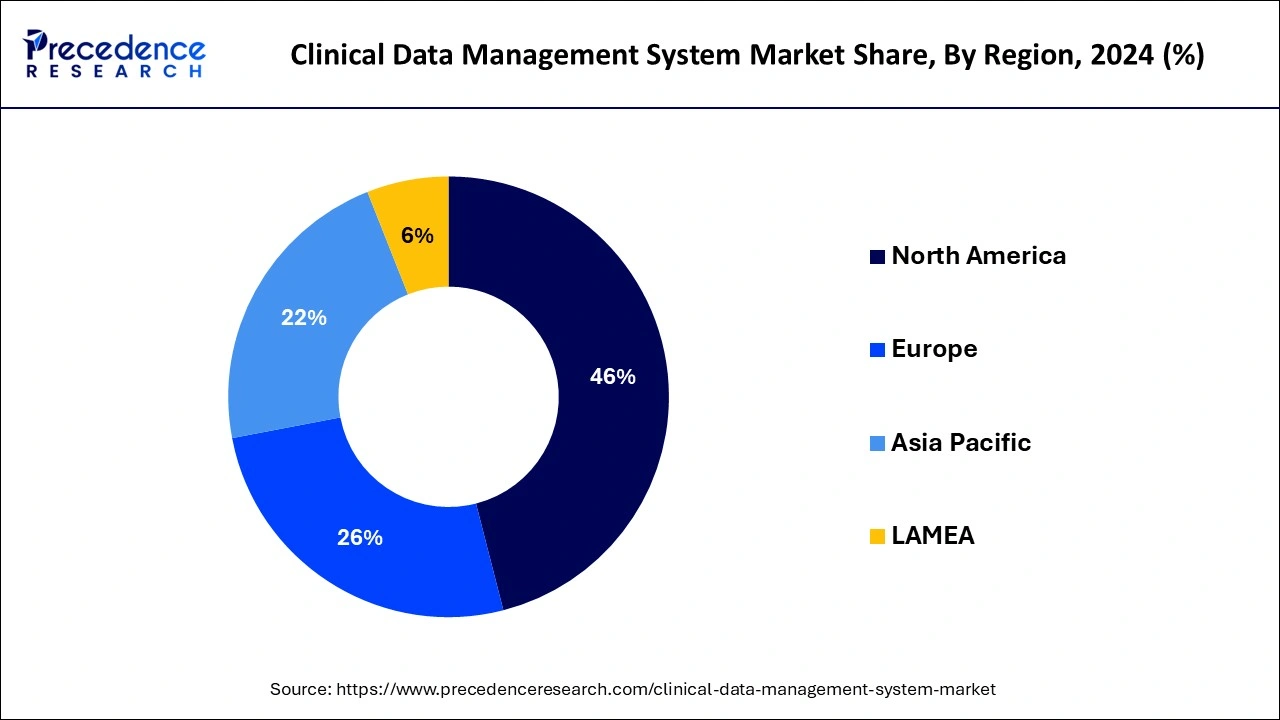
The growth of this industry is being fueled by the increasing need for clinical data management services. Some of the key factors driving this unprecedented demand are listed below.
1. Increase in Volume of Clinical Research
Pharma and biotech companies pursue trials more than ever before-from rare diseases to individualized cancer therapies. Presently, clinical pipelines are expanding, and global trials are also becoming complex.
As a result, clinical research data handling demands software that can adapt to multiple protocols, regions, and regulatory standards. They cannot be manually handled with spreadsheets and Excel anymore. Software needs to have intelligence, interoperability, and take into account auditability from day one.
2. Rise in Patient-Centric and Decentralized Trials
From being tested to supervising trials, patients are collaborators in the entire care mechanisms. With ever more health mobile software, wearable, and telehealth sceneries, trials are now moving outside the walls of clinics. That shift entails the new meanings behind the capturing and managing of trial data.
CDMS tools have the ability to promote remote data entry, real-time syncing, and multilingual interfaces,directed through custom clinical data management software development. This decentralization has transformed agile cloud-based platforms from just useful to absolutely vital.
3. Complexities of Multi-Source Data Integration
Healthcare data are messy, half-fragmented, and in multiple formats- laboratory data, electronic health records, gene sequence, and IoT device feeds. Hence, a modern clinical database software solution, alongside its facilities to store data, should harmonize the data intelligently.
This means that CDMS platforms are fast becoming engines for real-time data synthesis. It enables researchers to spot trends in the healthcare industry sooner and bring forward adaptive trial designs. They can identify red flags before they evolve into bigger problems, such as protocol deviations or regulatory bottlenecks.
4. Regulatory Pressures and Compliance Standards
Tightening is the global regulatory climate. Data integrity laws such as 21 CFR Part 11, HIPAA, and GDPR are making compliance more diligent and precise; hence clinical data platforms today must have robust audit trails with secure access layers and automated discrepancy resolution. Its healthcare data management at this level demands systems that regulators are confident about.
5. The Need to Accelerate Time-to-Market
Speed matters. Each day endured by a drug to hit the market means deferred patient access and absent revenue. Intelligent CDMS tools shorten trial time by way of automated queries, removal of redundancies, and integration with analytics dashboards. So it goes without saying that for both budding HealthTech firms and established Pharma giants, investing in powerful clinical data management software is the way to go.
6. Interdisciplinary Collaboration Across Stakeholders
In modern trials, data lives in shared ecosystems. From CROs and sponsors to regulation reviewers and data analysts, everybody needs to head into a clean set of common data. It is the reason why CDMS tools are now increasingly being developed by or in collaboration with expert tech teams. Also, in the present day, healthcare organizations now partner with top-rated mobile app development company to build solutions to streamline medical and healthcare operations.
Benefits of Clinical Data Management Software
The healthcare system has now become ever-accelerating and complex. It is a domain where precision matters a lot. And clinical data is something you have to manage very cautiously. Other benefits that a clinical data management platform confers include those mentioned below. These benefits alter the manner in which your healthcare or clinical organization approaches risk, compliance, and discovery.
1. Precision, Not Just Collection
Let me be clear: data collection in clinical trials is easy. Ensuring validity, consistency, and protocol compliance in thousands of entries from various sites and devices? Now, that’s the real hard part.
The clinical data management system does this validation automatically, signaling discrepancies, missing values, or entries outside the prescribed range before such instances become regulatory nightmares. In essence, real-time quality assurance is provided rather than retrospective.
2. Real-Time Visibility & Decision Making
Modern research ambiance demands speed and agility. Be it mid-trial adaptations, dosage adjustments, or pre-empting early safety signals with live clinical research data, it all boils down to how fast you can pivot. A modern clinical database software architecture enables real-time dashboards and real-time alerts and empowers a sponsor, CRO, or the investigator with insights on which to act immediately, not weeks later.
3. Built-In Compliance and Audit-Readiness
It isn’t enough to simply do things right; you must also be able to prove that you did so. CDMS platforms are designed with built-in logic trails, version tracking, and encryption protocols that seamlessly conform to FDA, EMA, and GDPR frameworks. Thus, it does not solely work as clinical data management. It is litigation-grade, regulator-grade, investor-grade confidence packaged into your daily workflows.
4. Allows the Scalability for Multi-Site & Multi-Phase Trials
As trial executions stretch beyond their size in geography and scope, the architectural presence of your data platform either becomes their catalyst or their bottleneck. The best systems are made with the consideration of growing with you.
It should be able to handle anything ranging from local pilot trials to global Phase III studies. The presence of flexible APIs and modular workflows allows for new study arms, data streams, or collaborators to be plugged in without performing a complete restructure of the entire pipeline.
5. Integrated With Healthcare Data Analysis
Data only has value when it is useful in making decisions. Thus, advanced platforms do not act as isolated silos but could integrate with analytics engines, AI tools, and visualization modules that support thorough healthcare data analyses. Clinical data management systems provide predictive insights, anomaly detection, and adaptive trial designs.
6. Greater Collaboration and Remote Availability
Modern clinical trials are collaborative ecosystems distributed across geographies and disciplines. Cloud-native platforms facilitate role access, which is multi-lingual and performs automatic task routing. Cross-accessibility is especially required for decentralized or hybrid clinical trials.
7. Efficiency Gains Executed While Return on Investment
Efficiency has always been the most important aspect for any healthcare or clinical organization to consider. A smart clinical trial data handling platform, from automated query management to integrated eCRFs and audit logs, can cut weeks from timelines and tens of thousands from budgets.
That ROI would be a bare reality to any research group operating under tight deadlines or a low budget. That is precisely when a custom clinical data management software solution is required that can efficiently manage all clinical data management services.
Read Also : 8 Future-Proof Healthcare App Ideas for Startups & Entrepreneurs
8. Future-Proofing Through Innovation
Since more and more research turns into decentralized research with wearables, genomic sequencing, and AI-driven insight, an urgent need now arises for an adaptable and ever-evolving data platform. Future will, therefore, be for platforms that are not built only for today’s trials but for tomorrow’s innovations. Thus, a well-architected clinical data management software or healthcare learning management system would be the bedrock for future breakthroughs.
Buying Off-the-Shelf vs Developing Custom Clinical Data Management Software: Which Approach Wins?
Here comes the tricky and confusing part in this journey. Generally, you get two options for a CDMS. One can purchase a ready-made CDMS or its paid subscription, or one can build one custom clinical data management software or invest in dedicated healthcare application development as per its own organization’s needs.
Now, every entry, every timestamp, and every protocol deviation is a node on this larger accountability tree. Hence, companies make a consulting decision when it comes to deciding whether to purchase an off-the-shelf clinical data management system or build it from scratch.
We all know what might affect the quality and efficiency so much. Therefore, to assist you in making this decision, here are both sides decoded at a functional, strategic, and economic level.
Off-the-Shelf Solutions: Affordable and Faster Time-To-Market
Buying an off-the-shelf clinical data management software sometimes feels like convenience being chosen over complexity. The immediate benefits? Quick deployment, compliance features built-in, and workflows that are tried and tested across multiple clinical environments. This option is certainly attractive for research teams with generic data needs, limited timelines, or smaller budgets.
But here’s where the problem lies: Off-the-shelf software is catered to the average, making the specific hard.
Plug in with a local lab’s diagnostic feed? Real-time integrations with the wearable device trial? Regulatory workflows by country? Either push the boundaries of customization or change your research protocol to fit the software, rather than the software adapting to you.
Custom-Built Solutions: Best Fit for Precision Clinical Data Science
Custom clinical data management software development, on the other hand, responds to your protocol logic, user behavior, and data flow definitions, be they for complex randomization algorithms, adaptive trial logic, or multiple-language investigator portals. Custom clinical data management software allows actually design the system along with your science.
This does take time and money upfront since you will have to hire a custom app development company to build a dedicated CDMS for your organization.
But for mid-to-large research groups or sponsors of multi-phase, multi-country studies, this investment pays off in precision, control, and long-term agility. Think not of it as building software but as building future-proof infrastructure for advanced clinical data research and management.
Head-to-Head Comparison
| Criteria | Off-the-Shelf CDMS | Custom Clinical Data Management Software |
| Speed of Implementation | Rapid deployment (weeks) | Longer timelines (months) |
| Cost (Initial Investment) | Lower upfront cost | Higher development cost |
| Flexibility & Scalability | Limited customization | Fully customizable and scalable |
| Compliance Readiness | Built-in regulatory features | Tailored compliance with full control |
| Integration Capability | Often restricted to vendor-approved APIs | Can integrate with any third-party systems |
| Protocol Complexity Handling | Best for standardized workflows | Ideal for complex trial structures |
| Vendor Lock-In Risk | High (dependent on updates/support) | Full ownership of code and roadmap |
| Long-Term ROI | Moderate (may need upgrades/customization) | High (built exactly to your evolving needs) |
So Which One Should You Go With?
If small trials are the target of your organization working in a low-regulatory-risk environment, then off-the-shelf software would give a stepping stone something that is ‘good enough’ yesterday.
In contrast, though, if your trials are complex, your workflows non-standard, or your data strategy is long-term-then building a platform with a healthcare app development company is not a luxury; it is a strategic necessity. Because in the world of clinical trials, efficiency is nice, but precision is what will help you win.
What Is the Cost of Developing a Custom Clinical Data Management Software?
Let me speak plainly, custom clinical data management software is not an off-the-shelf expense; it has to be treated as a strategic investment. In the course of pricing, it will depend not just on the number of features desired but on how much precision, scalability, and regulatory readiness are there in the system. As per 2025 estimates, here is a typical cost range for developing clinical data management software:
| Development Level | Description | Estimated Cost Range (USD) |
| Basic MVP | Ideal for small-scale or early-phase clinical trials. | $40,000 – $70,000 |
| Mid-Level System | Designed for multicenter studies or clinical teams | $80,000 – $150,000 |
| Enterprise-Level | Built for large-scale clinical research organizations. | $180,000 – $250,000+ |
So, based on the above table, the cost of clinical data management software development can fall between $40,000-$70,000 for a basic-level. However, you should also note that there are several factors that affect the overall cost, such as:
- Compliance Customization (HIPAA, GDPR, 21 CFR Part 11)
- Custom EDC & eCRF Design
- Integration with Clinical Labs, EMRs, or Wearables
- Real-Time Analytics & Dashboards
- Multilingual or Multisite Access Control
- Level of Security Architecture
Moreover, if you are working with a top-notch healthcare mobile app development company, then you have invested not just in code but also in domain knowledge and foresight in regulations and user-friendly design that doesn’t make your research team hate using the system.
While custom clinical data management software might be more expensive in the beginning compared to pre-made options, it yields a substantial ROI by adjusting to your research and regulatory changes and preemptively removing any data bottlenecks that threaten the integrity of your trials.
Conclusion
As the clinical landscape grows more complex, so must the tools that support it. Static spreadsheets and disconnected legacy systems no longer serve the purpose. What’s needed now is a dynamic, compliant, and scalable digital healthcare ecosystem that enables our clinical organization to make the most of its data.
But, since it requires a great technical expertise, you need assistance from an expert healthcare app development company that can assist you in building a clinical data management software exclusively designed for your clinical data management needs.
FAQs
Q1. What is the Clinical Data Management process?
The Clinical Data Management (CDM) process is a structured workflow designed to collect, validate, clean, and manage clinical trial data to ensure it is accurate, consistent, and statistically sound. It includes activities such as CRF design, database setup, data entry, validation, discrepancy management, coding (e.g., MedDRA, WHO-DD), and database lock, which make sure the data meets regulatory and scientific standards for analysis.
Q2. How do you manage Clinical Data?
Clinical data is managed through a combination of electronic data capture (EDC) systems, pre-defined validation rules, and real-time data review. Key steps include creating a data management plan (DMP), designing logical edit checks, ensuring compliance with regulatory standards (e.g., 21 CFR Part 11, GCP), handling queries effectively, and maintaining audit trails. Data is continuously monitored and cleaned until the final database lock for statistical analysis.
Q3. Why is CDM important in clinical trials?
CDM is critical because it safeguards the integrity and reliability of data that underpins clinical trial outcomes. Without a robust CDM process, inaccurate or incomplete data can lead to flawed conclusions, regulatory delays, or even trial rejection. Proper data management ensures compliance, protects patient safety, enables valid statistical analysis, and accelerates drug or device approval timelines.
Q4. What tools or software are used in Clinical Data Management?
Common tools used include Medidata Rave, Oracle Clinical, OpenClinica, REDCap, and Castor EDC. These platforms offer EDC capabilities, data validation, audit trails, coding integration, and query management. In addition, clinical data management software can be custom-developed to meet specific sponsor or regulatory requirements with enhanced control, scalability, and integration features.










 India
India USA
USA Australia
Australia Canada
Canada UK
UK UAE
UAE